
Resources > BLOG >
June 16, 2025
When preparing a regulatory submission for FDA approval, evidence is the foundation. It supports claims, informs regulatory decisions, and ultimately determines how quickly and confidently a device or drug moves through the approval process. But strong evidence alone is not enough. What happens when high-quality studies are buried in scattered files or unsupported by documentation?
How that evidence is organized, documented, and presented can be just as critical as the data itself. Disorganized or incomplete documentation, gaps in traceability, and inconsistent methodologies can all lead to delays, additional questions from regulators, or even rejections.
This article explores why evidence handling matters, what common challenges regulatory teams face, and how structured workflows and the right tools can support more confident, audit-ready submissions.
Why Evidence Handling Matters in FDA Submissions
Regulatory bodies such as the FDA expect more than just the presence of supporting scientific evidence; they look for clarity in how that evidence was identified, assessed, and applied. These principles enable reviewers to follow the decision-making process and evaluate the reliability of the conclusions presented. When evidence is poorly managed by being unstructured, inconsistently documented, or lacking a clear audit trail, it can slow down the review process. These delays can be costly, both in terms of launch delays and resource utilization.
Submission-ready evidence meets regulatory expectations by being:
When this level of organization is in place, teams are better prepared to deliver regulatory dossiers that meet FDA submission requirements with confidence.
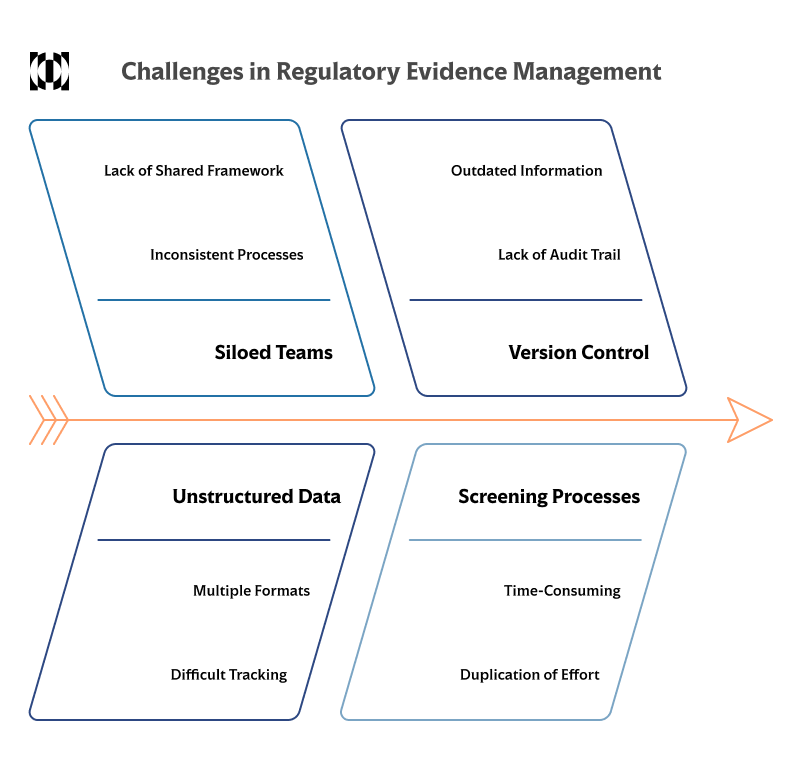
Common Challenges in Managing Regulatory Evidence
Handling regulatory evidence is often more complex than expected. Many teams face recurring obstacles that can compromise the efficiency and clarity of their FDA submissions.
One common issue is the presence of siloed teams working with inconsistent processes. Without a shared framework for collecting, screening, and documenting evidence, it becomes difficult to maintain consistency across submissions, which can lead to conflicting interpretations or missing information.
Another challenge is managing unstructured literature and data sources. Scientific studies, technical reports, and clinical findings are often stored in multiple formats and locations, making it difficult to track what has been reviewed and what remains outstanding.
Version control is also a frequent concern. Without a clear audit trail, teams can lose track of how decisions were made or which documents reflect the most current information. This lack of traceability increases the risk of errors during dossier preparation.
Finally, screening processes are often time-consuming and prone to duplication of effort. When teams work manually or without coordination, they may end up reviewing the same material multiple times or missing key studies altogether.
These challenges highlight the importance of structured, transparent workflows in regulatory evidence management.
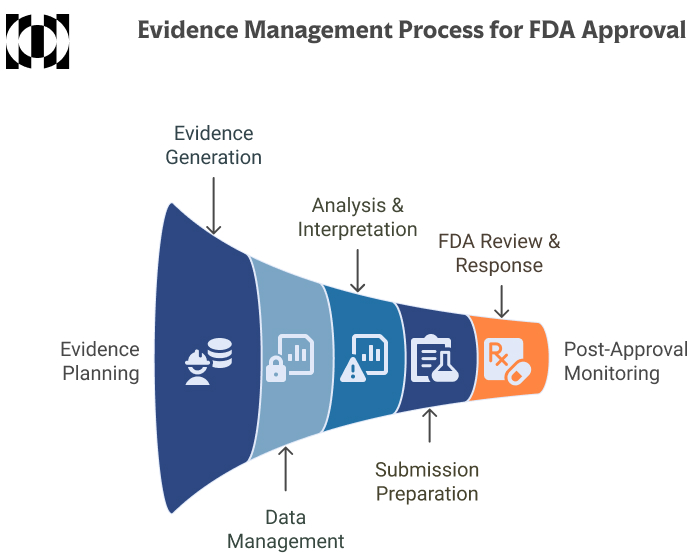
Characteristics of Well-Handled Evidence and the Role of Review Tools
Clear, well-organized evidence is central to a strong regulatory submission. While scientific quality is essential, regulators also expect structure, transparency, and traceability in how that evidence is gathered and presented. This means more than just compiling studies; it requires a consistent process for identifying, screening, summarizing, and documenting literature.
Well-handled evidence begins with the systematic collection of data using predefined methods and clear inclusion criteria. From the first search to the final selection, each step should be documented in a way that allows others, both within the team and at regulatory agencies, to understand what was reviewed and why certain sources were included or excluded.
Screening decisions should also be transparent. When teams record the rationale behind each inclusion or exclusion, they create a foundation for stronger conclusions and fewer questions during the review process. A traceable path from each claim back to its source study strengthens reproducibility and provides credibility.
A regulatory dossier should be easy to navigate, internally consistent, and aligned with the expectations of reviewers. Disorganized or undocumented workflows increase the risk of delays or rework, which can impact both timelines and resource utilization.
Supporting this level of structure often requires more than a manual approach. Systematic literature review platforms such as Laser AI can help teams manage complex evidence workflows by improving the efficiency of screening, maintaining a clear audit trail, and facilitating collaboration between scientific and regulatory staff. These tools assist with the process by helping teams organize and document their work in a way that meets regulatory standards.
By combining sound methodology with the right evidence synthesis tools, teams are better equipped to deliver evidence that is not only scientifically sound but also regulatory-ready.
What to Look for in a Systematic Literature Review Tool
When selecting a solution to support your evidence review process, consider the following key features:
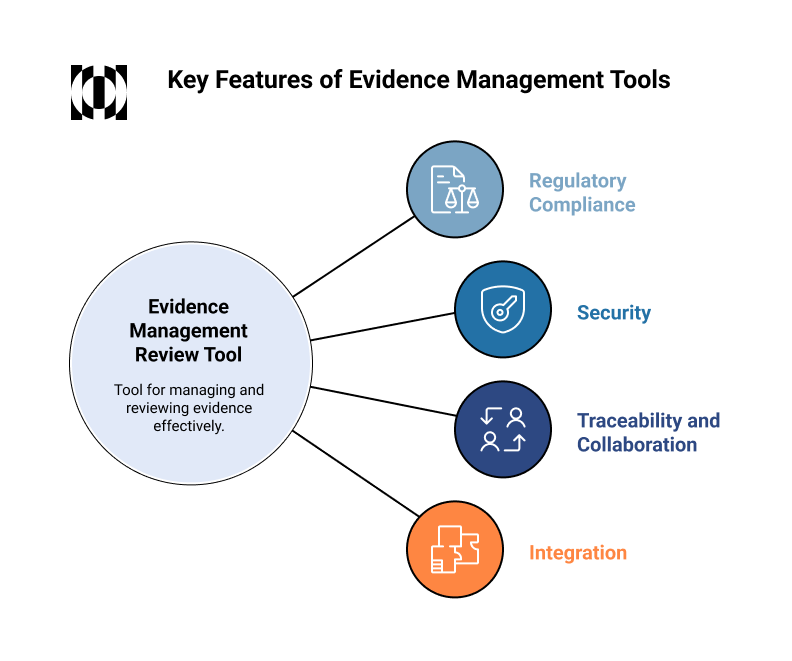
A strong FDA submission depends not only on the quality of the evidence itself but also on how that evidence is managed. These capabilities help ensure that your data is not only well-managed but also prepared for regulatory scrutiny and review.
Investing in better evidence workflows is more than a compliance exercise. It is a strategic step that can reduce delays, strengthen submissions, and support timely approvals.
To see how Laser AI supports clinical and regulatory teams in organizing and documenting evidence for FDA submissions, book a demo.
Related webinars:
Related blog posts: