Resources > WEBINARS >

Updated FDA guidance impacts medical affairs and off-label communications. Learn how AI tools like Laser AI ensure scientific soundness and compliance in proactive scientific exchange.
With the recent updates to the FDA's communication guidance, medical affairs professionals are facing an important shift from reactive information sharing to proactive scientific exchange. Our past webinar and this post delve into these changes, exploring how AI tools like Laser AI can help companies effectively disseminate scientifically sound information in a compliant manner.
Kasia Hein-Peters, a physician, brings a wealth of experience from years in the pharmaceutical and medical device industries. She is the founder of Abante Scientific, a consultancy dedicated to providing strategic guidance, medical affairs expertise, and market access solutions within the life sciences sector. In this discussion, Kasia shares her insights on the FDA communication guidance, particularly how these new regulations change medical affairs communications and the role of scientific soundness.
Read our post here or watch the webinar on YouTube
The FDA's approach to off-label communication has undergone many changes over approximately 15 years. This journey began around 2009 with the "good reprint practices guidance," which established a limited pathway for pharmaceutical and medical device companies to distribute medical journal articles about unapproved uses of their products. The initial guidance was updated multiple times before the release of the "final guidance" in January. This latest guidance addressed previous concerns, particularly the vague definition of "clinically relevant requirements," shifting the focus more towards "scientific soundness" in communications.
In the latest guidance, the FDA has changed three particular areas:
Kasia Hein-Peters suggests that the removal of the "clinically relevant requirements" actually clarified the issue, as clinical relevance is ultimately a judgment left to practicing physicians based on the best available evidence. This historical progression reflects a movement towards allowing healthcare professionals to utilize a broader range of scientifically sound information when making clinical decisions.
The FDA's intent with these changes is to return to evidence-based medicine principles, where physicians use the "best available evidence" to make clinically relevant decisions. The guidance allows for a broader range of scientifically sound studies and formats beyond just peer-reviewed randomized clinical trials, aiming to provide healthcare professionals with more comprehensive information to assess and utilize for patient care.

For compliant communications under the new FDA guidance, several critical considerations must be adhered to. All communications, regardless of format, must be truthful, not misleading, and provide sufficient information to allow healthcare providers to thoroughly assess the strengths, weaknesses, validity, and clinical utility of the scientific data presented. Transparency is key, especially for company-created materials, requiring appropriate disclaimers, clear indication of the product's regulatory status, and a very transparent presentation of any study limitations and uncertainties. Furthermore, source publications must always remain accessible. While the guidance is "digital-friendly," companies must be cognizant that platforms with character space limitations may not be suitable if they prevent comprehensive disclosure and context requirements, meaning the chosen platform must be appropriate for the depth of communication.
The concept of "scientific soundness" is central to these new guidelines. Kasia Hein-Peters emphasizes that this involves following generally accepted scientific principles for study design and methodology. Communications must provide a clear description of the stated hypothesis and the results, alongside an acknowledgement of all potential biases. Crucially, they must ensure the reliability and validity of the data, including adherence to protocols and statistical analysis plans established before the study. Ultimately, scientific soundness means presenting all necessary information to healthcare professionals, allowing them to make informed decisions based on the "best available evidence," which may include various study types beyond just randomized controlled trials, depending on the clinical context and the nature of the condition, such as orphan diseases.

The updated FDA guidance presents both opportunities and challenges for medical affairs, particularly with its emphasis on "scientifically sound studies," transparency, and continuous monitoring. The good news? AI tools like Laser AI are stepping up to bridge the gap, changing how medical affairs teams can meet these rigorous demands efficiently and compliantly.
Artur Nowak, founder of Evidence Prime, the developer of Laser AI, highlights how Laser AI directly addresses key areas of the new guidance:
The FDA now requires a comprehensive background of evidence, including not just the primary study but also conflicting data and limitations. This demands a thorough literature review. AI helps this process by:
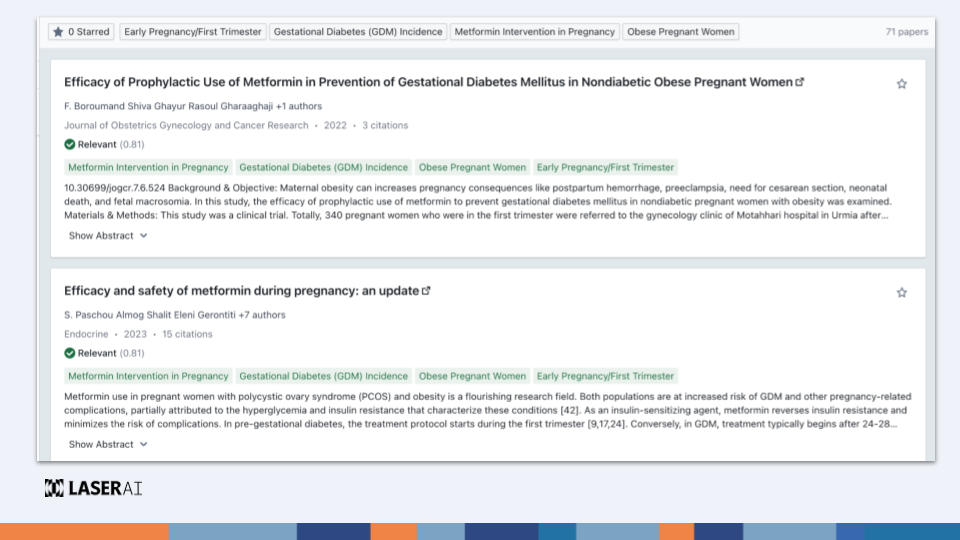
The guidance emphasizes the need to assess study design, methodology, and the overall reliability of data. Laser AI simplifies this complex task:
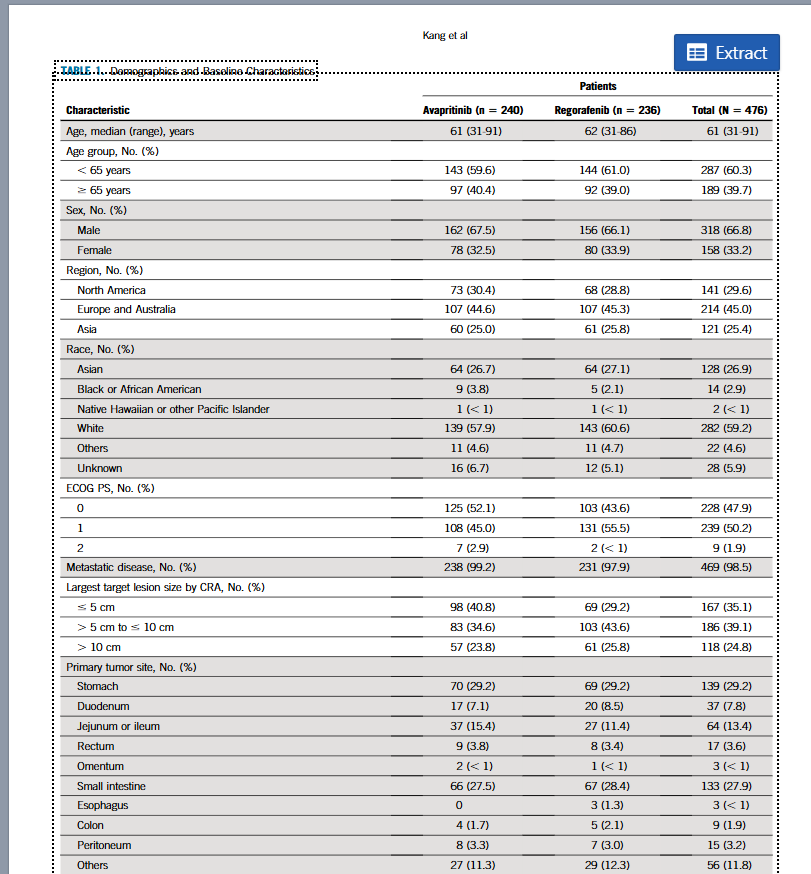
The FDA emphasizes that scientific knowledge is dynamic, requiring organizations to monitor for new studies, refutations, or retractions continuously. This means communication isn't a one-and-done task. Laser AI is built for this ongoing need:
In essence, Laser AI provides medical affairs professionals with the robust, auditable, and efficient tools necessary to not only meet the demands of the new FDA communication guidance but to truly embrace a proactive, evidence-based approach to scientific exchange.
This webinar and discussion has illuminated the critical shift in medical affairs communications driven by the new FDA guidance. The intent is clear: to return to the principles of evidence-based medicine, empowering healthcare professionals with access to a broader range of scientifically sound data to inform their clinical decisions. This move from reactive information sharing to proactive scientific exchange demands a new approach, one that prioritizes transparency, scientific soundness, and continuous monitoring.
As we've seen, AI can play an important role in navigating these complexities. Tools like Laser AI are not merely about enhancing efficiency; they are fundamental to ensuring compliance and maintaining the integrity of scientific communications. By facilitating structured data extraction, rigorous evaluation of study methodologies, and continuous surveillance for new evidence or retractions, AI provides the robust, auditable processes required to meet the FDA's expectations.
If you would like to see how AI can help your medical affairs communications? We encourage you to explore the capabilities of Laser AI. You can watch a demo of the tool here:
WATCH DEMO
Or book a call with our sales team.
Calendly Link from Kirsty
Speakers: